Diagnosing early onsets of dementia can be a challenge for clinicians. Current diagnostic tools usually cannot identify people in the early stages of disease. By the time patients receive a diagnosis, neurons have died and the brain may be well into the process of atrophy and inflammation.
Biomed Digital, LLC aims to leverage wearable technologies for biodata collection in conjunction with the powerful deep learning capabilities of machine learning. By gathering data from people not yet showing obvious clinical symptoms of cognitive decline, these devices could one day be used to create a digital phenotype that helps clinicians diagnose dementia early, before neuronal death.

Our team seeking to develop machine learning algorithms trained on a substantial quantity of clinical datasets to distinguish which individuals had cognitive impairment and which were healthy, based solely on the pattern of digital data received from the participants’ wearable devices and their responses to simple iPad tasks.
While in its early stages, further development of this data driven approach may yield substantial benefits for patients and medical providers in aiding diagnostics of Alzheimer's disease.
We follow strict privacy and security measures to protect consumer data such as the US Health Insurance Portability and Accountability Act of 1996 (HIPAA) to protect health information in electronic form.
For further information about our products, please contact us. Contact us
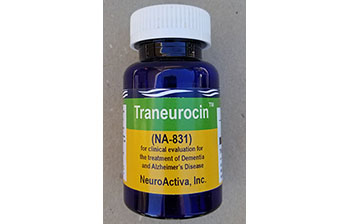
Traneurocin™ or NA-831 provides neuroprotection and catalyzes the regeneration of new neurons. Oral 30 mg per day for patients with early onset of Alzheimer’s Disease. NA-831 is manufactured by NeuroActiva, a subsidiary of Biomed.
Learn more
Biomed Digital is harnessing the power of wearable technology and machine learning to create a digital phenotype that helps clinicians diagnose early onset of Alzheimer’s disease.
Learn more
Biomed is conducting Phase 3 clinical trials of NA-831 for the treatment and prevention of Alzheimer’s disease. We also plan Phase 2/3 trials of NA-831 in combination with Atazanavir, Remdesivir, and Dexaneurosone and Ivermectin for treating Covid-19
Learn more
An injectable drug designed to stimulate neurogenesis and reduce muscular spasticity, a candidate for treatment of Alzheimer’s disease and Parkinson’s disease. NA-704 can be administered with the MICROS. NA-704 is in the Phase 1 safety studies.
Learn more
The MICROS is a controlled release infusion system which has been approved for marketing in the US. It is used for intravenous administration of injectable drugs. NA-704 can be administered via the MICROS at home care settings. It is an accurate, safe and cost effective drug delivery system.
Learn more
In addition to Alzheimer's Disease, NA-831 shows promise as a treatment for MDD due to its neuroprotective properties. Results of a Phase 1B study have demonstrated a clean safety profile with no adverse side effects.
Learn more